- Research
Engineering Copper surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons
In this study we control the surface structure of Cu thin-film catalysts to probe the relationship between active sites and catalytic activity for the electroreduction of CO2 to fuels and chemicals.
Hahn, C., Hatsukade, T., Kim, Y.-G., Vailionis, A., Baricuatro, J. H., Higgins, D. C., Nitopi, S. A., Soriaga, M. P., and Jaramillo, T. F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.1618935114 (2017)
Anthropogenic global warming necessitates the development of renewable carbon-free and carbon-neutral technologies for the future. The electrochemical reduction of CO2 offers sustainable routes for the production of fuels and chemicals. The development of active and selective catalysts for this reaction remains a major challenge. Out of the polycrystalline metals, Cu is the only one that shows propensity for the formation of products that require more than two electrons (>2e− products), e.g., methane, ethanol, and ethylene, at considerable rates and selectivity. (1) Although polycrystalline Cu is not particularly selective toward any one >2e− reduction product, (2) recent work has demonstrated that atomic-level structural modification of Cu(pc) into Cu(511) leads to the generation of ethanol as the sole product upon reduction of CO at low potential. (3) Thus, it is critical to understand active site motifs associated with this unique selectivity and to apply this knowledge in the rational design of new electrocatalytic materials.
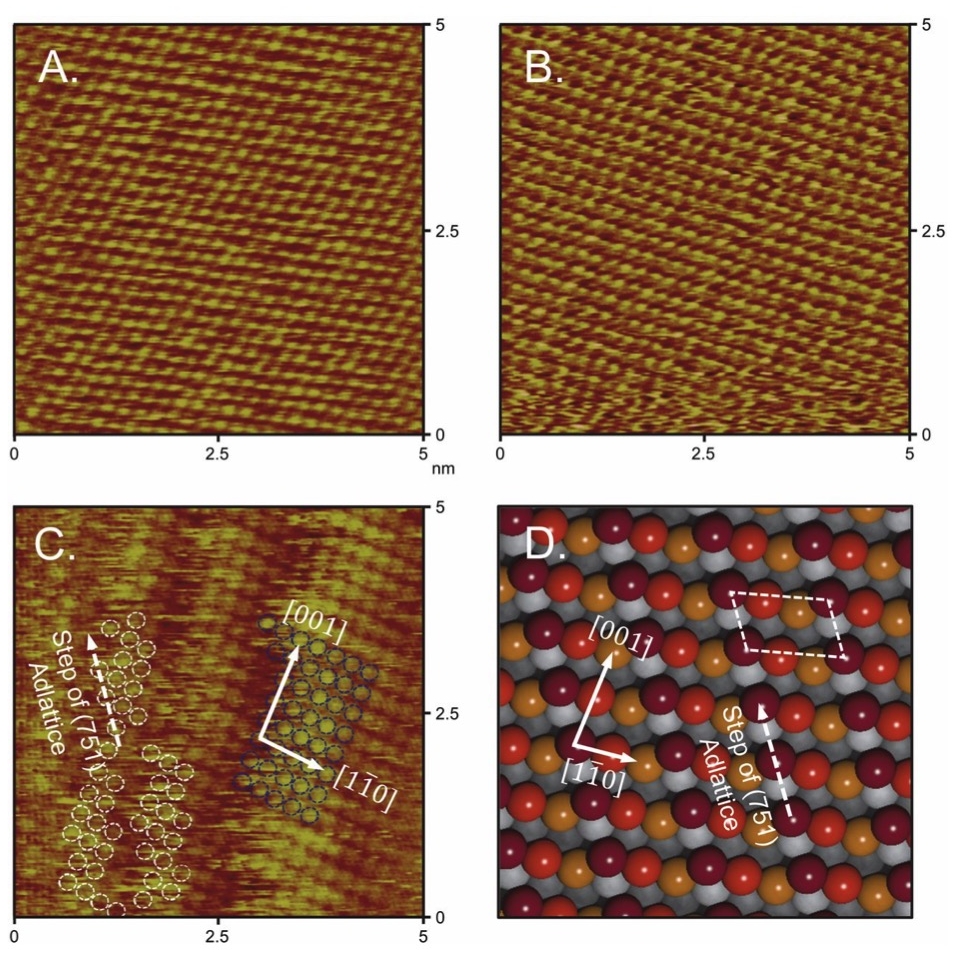
Atomically resolved in situ ECSTM images of (A) Cu(111), (B) Cu(100), and (C) Cu (751) thin films. (D) An ideal atomic model of the Cu(751) surface is used to compare step orientations. Reprinted from Hahn et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. PNAS 114(23), 5918-5923, DOI: 10.1073/pnas.1618935114 (2017).
In this study, the surface structure of Cu thin-film catalysts was examined to probe the relationship between active sites and catalytic activity for the electroreduction of CO2 to fuels and chemicals. (4) Cu thin films were prepared on large-format (∼6 cm2) single-crystal substrates via physical vapor deposition, and epitaxial growth in the <100>, <111>, and <751> orientations were confirmed using X-ray pole figures. To understand the relationship between bulk and surface structures, in situ electrochemical scanning tunneling microscopy (ECSTM) was conducted on Cu(100), (111), and (751) thin films. The study showed that Cu(100) and (111) have surface adlattices that are identical to the bulk structure, and that Cu(751) has a heterogeneous kinked surface with (110) terraces that is closely related to the bulk structure. Electrochemical CO2 reduction testing revealed that whereas both Cu(100) and (751) thin films are more active and selective for C–C coupling than Cu(111), Cu(751) is the most selective for >2e− oxygenate formation at low overpotentials. The present results demonstrate that epitaxy can be used to grow single-crystal analogous materials as large-format electrodes and provide insights on how to improve selectivity and energy efficiency toward more valuable CO2 reduction products.
Additional mechanistic details can be unveiled from future work that controls the ratio of step and terrace sites on well-defined surfaces. Experiments that examine fine surface structural nuances under operando conditions, such as ECSTM and grazing-incidence X-ray diffraction, will be conducted to further explore the relationship between surface structure and catalytic activity. Ongoing work has used epitaxial Cu films as platform for the investigation of the promotion effects of alkali-metal cations on CO2 and CO reduction. Advancements in epitaxial growth will spur efforts in materials discovery studies on well-defined multi-metallic structures such as alloys and overlayers.
References:
1. Hori, Y., Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry, Vayenas, C. G.; White, R. E.; Gamboa-Aldeco, M. E., Eds. Springer New York: New York, NY, 2008; pp 89-189.
2. Kuhl, K. P.; Cave, E. R.; Abram, D. N.; Jaramillo, T. F., New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5 (5), 7050-7059.
3. Kim, Y. G.; Javier, A.; Baricuatro, J. H.; Soriaga, M. P., Regulating the Product Distribution of CO Reduction by the Atomic-Level Structural Modification of the Cu Electrode Surface. Electrocatalysis-Us 2016, 7 (5), 391-399.
4. Hahn, C.; Hatsukade, T.; Kim, Y.-G.; Vailionis, A.; Baricuatro, J. H.; Higgins, D. C.; Nitopi, S. A.; Soriaga, M. P.; Jaramillo, T. F., Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proceedings of the National Academy of Sciences 2017, www.pnas.org/cgi/doi/10.1073/pnas.1618935114.